By YAN Fusheng
For years, the study of senescent cells resembled a molecular guessing game. Researchers knew these dormant cells played paradoxical roles—some secreting inflammatory signals that accelerated tissue damage, others releasing factors to restrain it. Yet traditional tools could only lump all senescent cells into a single category. This blindness persisted until 2024, when Dr. ZHOU Bin’s team at the Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Chinese Academy of Sciences, redefined the rules of engagement.
Their breakthrough, published in Cell, came in the form of a genetic “Swiss Army knife”—a toolkit that acts as both microscope and scalpel, allowing scientists to track, delete, or reprogram senescent cells based on their identity. Suddenly, the cellular battlefield shifted from fog-of-war chaos to high-definition clarity. Scientists could now distinguish destructive “arsonists” from reparative “firefighters”. By selectively deleting the former population, the team achieved what earlier methods couldn’t: a 40% reduction in liver scarring.
The Tools: Masters of Cellular Fate
The heart of this breakthrough lies in three cleverly designed genetic tools. First, there’s Sn-pTracer, a sort of time machine that tags aging cells carrying the p16Ink4a gene—a major player in senescence—so researchers can watch their journey unfold over weeks. Then there’s Sn-cTracer, a genetic sniper that wipes out specific types of aging cells with a dose of diphtheria toxin. Finally, Sn-gTracer acts like a molecular editor, tweaking genes inside these cells to see if the troublemakers can be turned into lifesavers.
These tools use a clever dual-recombinase system—think of it as a two-step security check—to zero in on the right cells without collateral damage.
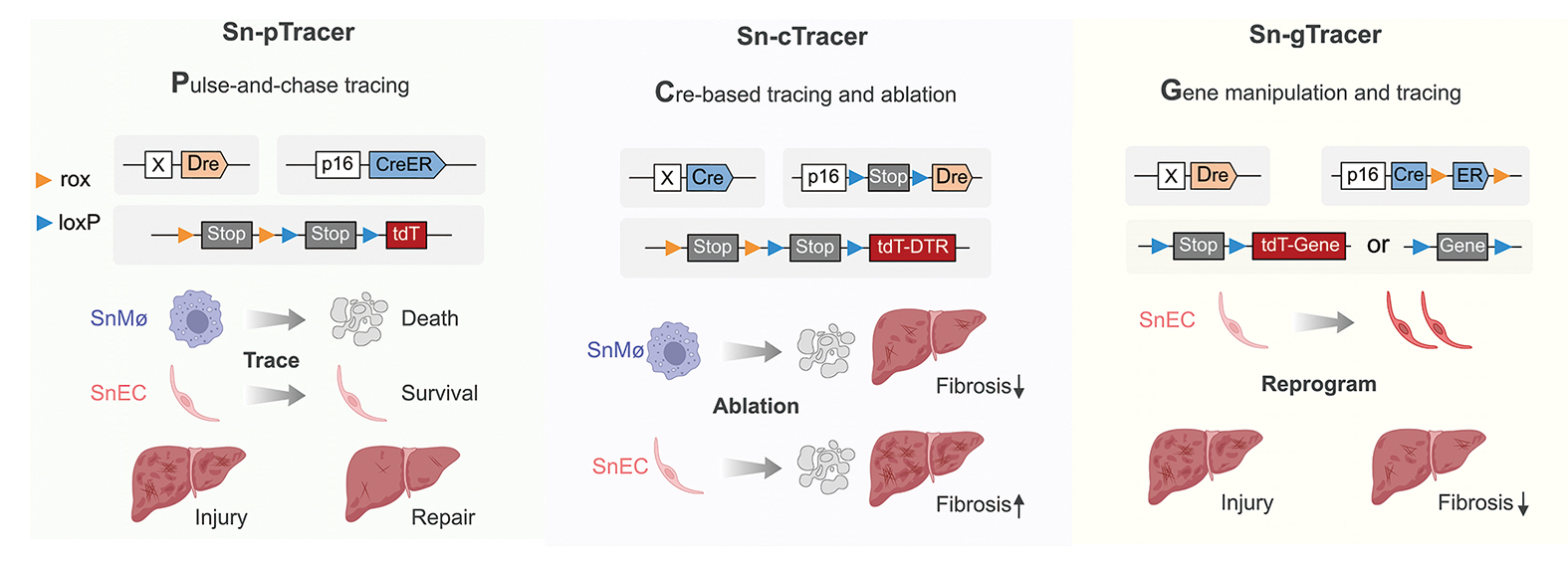
The three key genetic strategies used to study the fate and function of specific p16Ink4a+ cell types in liver fibrosis.
(Graphic: Adapted from Zhao et al., 2024)
Liver Fibrosis: A Drama in the Cells
The team tested their tools in mice with liver fibrosis, a condition affecting 100 million people globally. Using single-cell RNA sequencing and fluorescent tracers, they uncovered a cellular drama.
The villains turned out to be senescent macrophages—immune cells that, like reckless firefighters, flood the liver with inflammatory chemicals, fanning the flames of scar tissue. When the researchers used Sn-cTracer to take these troublemakers out, fibrosis dropped by an impressive 40%. It was like kicking the arsonists out of a blazing forest.
But then came the surprise heroes: senescent endothelial cells, which line the liver’s blood vessels. These cells usually help with repairs, but when they age, their powers fade. Deleting them made fibrosis worse, proving they’re not the enemy. However, when the team used Sn-gTracer to crank up a gene called Kdr—vital for fixing blood vessels—these cells roared back to life, battling fibrosis like champions.
Why One-Size-Fits-All Senolytics Could Backfire
The study shatters the myth that all senescent cells are harmful. Blindly eliminating them could be like bombing a city to stop a riot, as indicated by the study. For example, macrophage ablation increased anti-fibrotic T and NK cells, while endothelial cell removal triggered a surge in scar-forming mesenchymal cells.
These intertwined cellular dynamics explain why past senolytic (senescence-targeting) drugs showed mixed results. The discovery suggests that we need GPS-guided therapies—target the bad and spare the good.
Beyond the Liver: A Glimpse of What’s Next
The implications ripple beyond the liver. The tools could unravel senescence’s role in Alzheimer’s, atherosclerosis, and even longevity. But there are hurdles ahead. Even the sharpest tools might accidentally nick healthy cells. Moreover, mice aren’t humans—further confirmation in humans is required. To speed things up, the team has open-sourced their models via a Material Transfer Agreement, inviting global collaboration to accelerate discovery.
This research isn’t just about liver disease—it’s a manifesto for precision aging biology. By mapping senescence cell by cell, scientists are learning to harness its duality. For now, the liver’s silent war continues. But with these tools, science has drafted its first battle plan.
Reference
Zhao, H., Liu, Z., Chen, H., et al. (2024) Identifying specific functional roles for senescence across cell types. Cell, 187(25), 7314–7334.e7321. doi:10.1016/j.cell.2024.09.021

