Brain calcification has aging-associated prevalence that increases from ~1% in young people to more than 20% in the elderly. Patients with bulk brain calcification usually present progressive movement disorders (dystonia, ataxia, parkinsonism, chorea), neuropsychiatric symptoms and cognitive dysfunction. Identification of the genetic basis using pedigrees of primary familial brain calcification (PFBC), characterized by bilateral pathological calcium deposits in the basal ganglia and other brain regions, has been attracting increasing research attention and expanding the fundamental understanding of brain calcification in recent years. Since the finding of SLC20A2 as the first PFBC-associated gene by Prof. LIU Jingyu from Huazhong University of Science and Technology in 2012, three other genes including PDGFRB, PDGFB and XPR1 were found to be associated with PFBC in the following years. However, the genetic causes of the rest 50% families remain unidentified.
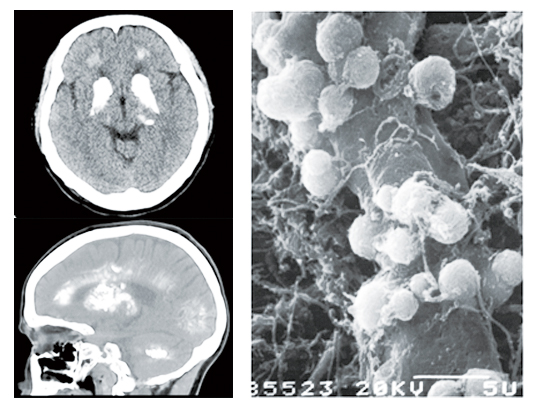
Left: Horizontal (top) and sagittal (bottom) view of computed tomographic (CT) imaging of calcification deposits in the brain of a PFBC patient. Right: Sphere-shaped calcification deposits distributed around blood vessels in the brain shown by scanning electron microscope (SEM). [This SEM image is adopted from Kobayashi,S.,et al.,1987. Acta Neuropathol 73(1):62–66.]
A recent study published in Neuron demonstrated that mutation of MYORG gene is a new causative factor for primary familial brain calcification. This finding resulted from a joint work by Prof. CHEN Wanjin and Prof. WANG Ning’s team from The First Affiliated Hospital of Fujian Medical University (FMU) and Dr. XIONG Zhiqi’s lab at the Institute of Neuroscience (ION), Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences (CAS). This work identified loss of function mutation in MYORG gene as a new genetic cause of PFBC in a recessive pattern, and the development of calcification deposition in the brain of Myorg homozygous knockout mice confirmed this association.
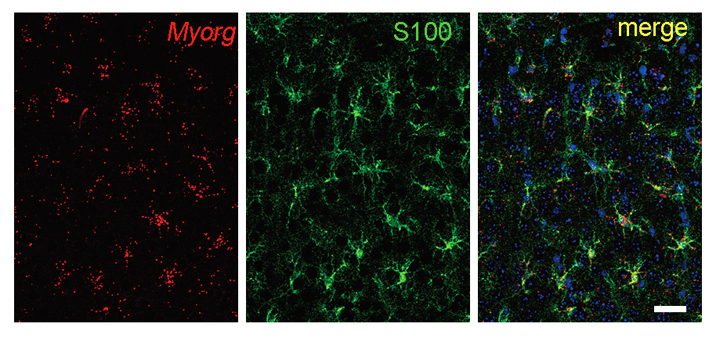
Co-localization of MYORG (in situ, red) and S100b (IHC, green) in the cerebral cortex of two-months-old mouse brain.
The FMU team led by Profs. CHEN Wanjin and WANG Ning took years’ effort and collected tens of PFBC families that distributed all over the country. Using whole exosome sequencing and Sanger sequencing, they found six PFBC families co-segregated with mutations in MYORG gene, which was also known as KIAA1161 or NET37, locates at 9p133, and encodes a protein product with 714 amino acids that belong to glycosidase subfamiliy 31. Before this report, there is completely no research finding that indicate a link of MYORG to brain calcification.
By joining forces with Dr. XIONG Zhiqi’s lab at ION, the joint team found a selective expression of Myorg gene in S100b-positive astrocyte by in situ hybridization, and the complete overlap expression of GFP that knocked-in after endogenous Myorg promoter with S100b-positive astrocyte reconfirmed this specific expression pattern. Functionally, development of calcification deposits was found in brains of mice at an age over 8-months, which showed a similar element composition including calcium, phosphate and oxygen as that from human patients, suggesting that loss-of-function of Myorg is a causative factor of brain calcification.
The identification of MYORG as the first autosomal recessive gene for PFBC, and that mutation of this gene accounts for 11% of total PFBC families and 50% of recessive families, suggests MYORG mutation is a critical genetic factor of PFBC. Moreover, the selective expression of MYORG in astrocytes demonstrates that astrocyte is a new cell type involved in brain calcification. In addition to pericytes, in which deficiency of PDGFB-PDGFRB signaling causes brain calcification, astrocytes are also an essential component of the blood-brain barrier (BBB) both in human and rodent brains. This finding thus establishes a new keystone for the shaping of the cellular pathological theory of brain calcification.
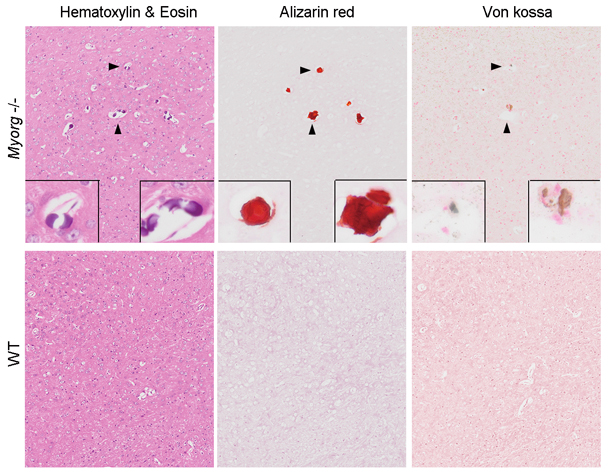
Visualization of calcification deposits by HE, Alizarin red or Von kossa histological staining in the brain of Myorg-knockout mouse.
This work was equally contributed by Dr. YAO Xiangping (FMU), Dr. CHENG Xuewen (ION), Dr. WANG Chong and Ms. ZHAO Miao, who were led by Prof. CHEN Wanjin (FMU), Dr. XIONG Zhiqi (ION) and Dr. WANG Ning (FMU). Dr. HU Qian from the Imaging facility, as well as Miss CAI Yijun from the animal facility (ION, CAS) provided the technical assistance. This work was supported by grants from the National Natural Science Foundation of China (NSFC), CAS Pilot Program, the Ministry of Science and Technology of China, the National Key Clinical Specialty Discipline Construction Program of China and the Key Clinical Specialty Discipline Construction Program of Fujian Province, China.

