By YAN Fusheng
Beneath the soil, roots don’t just soak up water and nutrients—they actively recruit and organize their microbial companions. New research reveals how a tiny amino acid leak can shape underground communities, with implications for plant health and agriculture.
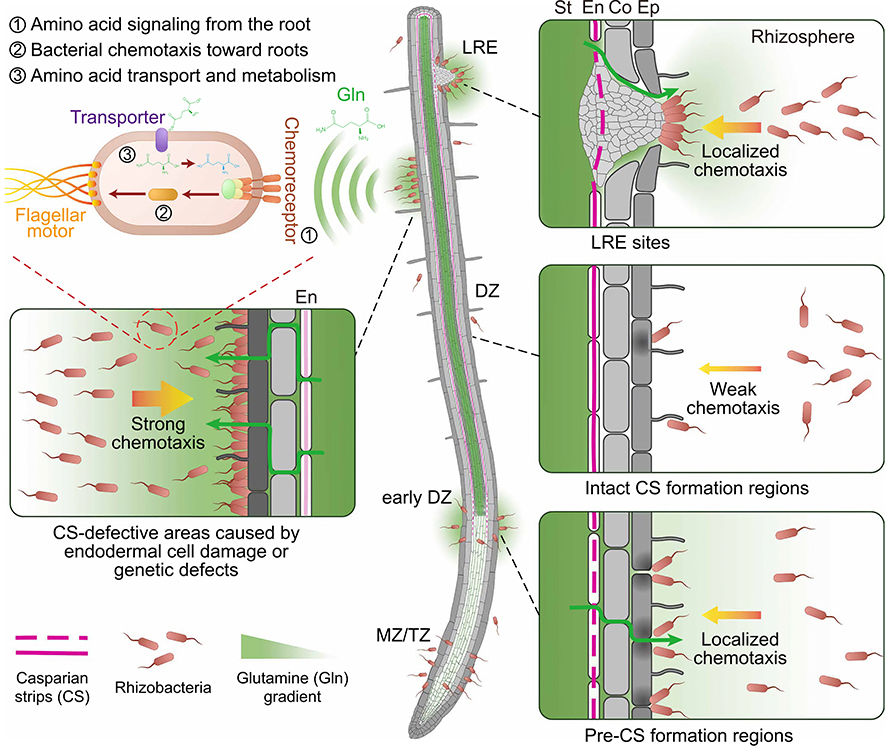
Plant roots leak amino acids at vulnerable spots, creating chemical trails that lure beneficial bacteria to precise locations where they aid nutrient uptake and defense. (Credit: Tsai et al., 2025)
In the silent, complex world beneath our feet, a delicate dance dictates plant health—the selective recruitment of soil bacteria to form a specialized root microbiome, known as the rhizosphere. These microscopic communities are not merely passive residents but are fundamental allies, influencing root development, bolstering plant health, and conferring resilience against both biotic and abiotic stresses.
Scientists have long known that this alliance is brokered through root exudates, a rich mix of organic molecules secreted by plants into the soil. However, one puzzle remains: exactly how, when, and where do roots release these invisible invitations to their microbial guests?
Now a team led by Dr. ZHOU Feng at the CAS Center for Excellence in Molecular Plant Sciences and Dr. Niko Geldner at the University of Lausanne in Switzerland developed a “mini-hydroponic system” to tackle this challenge. This miniature setup enables high-resolution observation of bacterial spatial distribution and colonization dynamics on young roots, revealing how metabolites shape microbial communities.
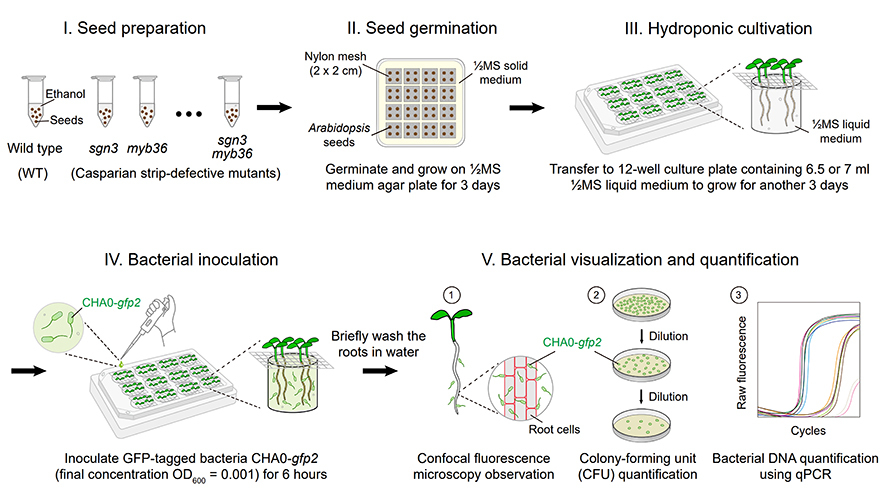
Schematic representation of the mini-hydroponic system setup developed for studying initial bacterial colonization on Arabidopsis roots. (Credit: Tsai et al., 2025)
Using this system, the joint team discovered that plant roots function as sophisticated security checkpoints, using controlled glutamine leakage to orchestrate microbial spatial settlement in the soil. Published in Science on October 2, 2025, the work illuminates how plants map and manage their underground microbiome.
The Gatekeeper
At the heart of this biological “border control” lies the endodermis—a cellular layer that acts as a selective gatekeeper between the soil and the plant’s inner tissues. Like tight junctions in the mammalian gut, the endodermis regulates the uptake of water and nutrients while preventing valuable metabolites such as sugars, organic acids, and amino acids from leaking back into the soil. Its effectiveness depends on the Casparian strip, a lignin-based seal that fortifies the spaces between endodermal cells.
The team noticed that bacterial colonization on Arabidopsis thaliana roots peaked where this barrier was weakened or temporarily breached. The commensal or beneficial bacterium Pseudomonas protegens CHA0 preferentially settled at lateral root emergence (LRE) sites, where the endodermis naturally ruptures during root branching. Plants carrying severe Casparian strip defects—such as the sgn3 myb36 mutant—showed widespread colonization along mature root zones. These patterns pointed to a compelling idea: microbes might be drawn to the nutrients—leakage of metabolite of the plant during the transient lapses of its inner barrier.
The Beacon and the Feast
Through sophisticated untargeted metabolomic analysis of root exudates, the researchers pinpointed the molecule acting as the microbial magnet. Exudates from plants with compromised endodermal barriers (sgn3 myb36 and rbohf mutants) showed a striking surge in amino acids compared with wild-type roots—dominated overwhelmingly by glutamine, which accounted for more than 80% of total amino acid leakage.
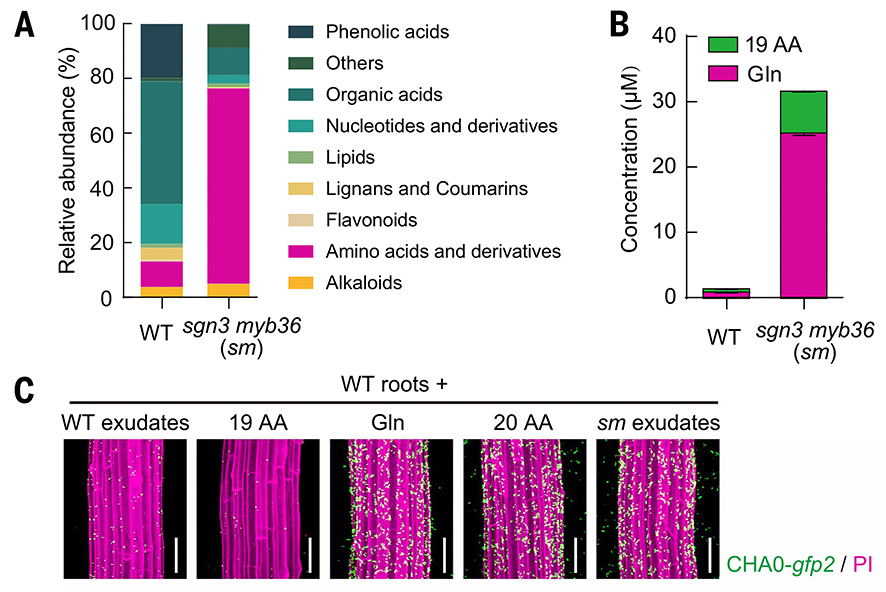
Metabolomic profiling (A) showed root exudates from sgn3 myb36 mutants were dominated by amino acids. Quantitative analysis (B) confirms glutamine (Gln) as the major leaked compound, far exceeding the combined pool of the remaining 19 amino acids (19 AA). When wild-type roots are supplied with Gln or mutant exudates, Pseudomonas protegens CHA0 rapidly accumulated along roots, demonstrating glutamine leakage fuels bacterial colonization (C). (Credit: Tsai et al., 2025)
Ordinarily a key transporter of nitrogen to the shoots, glutamine here might take on a dramatic double life, reckoned the researchers. It might serve as a chemical beacon guiding bacteria toward the root, and meanwhile as a nutrient source fueling the rapid growth of the latter. Bacterial buildup, occurring within just minutes around defective roots, might indicate active chemotaxis, given that it happens at a rate even quicker than bacterial doubling and hence impossible to be explained by proliferation alone. This assumption was confirmed when they observed in experiments that chemotaxis-impaired Pseudomonas protegens mutants failed to home in on glutamine-rich sites or accumulate at natural “entry points” such as lateral root emergence zones.
Moreover, the team found that glutamine doesn’t just lure bacteria—it defines the spatial pattern of microbial colonization. When wild-type plants were supplemented with glutamine, bacterial clustering occurred subsequently, mimicking what could be seen when the plant carried the above-mentioned barrier mutants. These hotspots form what the researchers describe as “short-lived, spatially confined metabolic niches” that effectively seed the root microbiome: In healthy plants, such leakage is brief and precisely localized, occurring only where new root structures are forming, or endodermal seals are not yet complete.
The Trade-Off
This precise control over metabolite leakage has profound physiological consequences. The study reveals a key trade-off in how plants manage their subterranean ecosystems: balancing microbial cooperation with defense.
When the endodermal barrier fails, as in the sgn3 myb36 mutant, chronic leakage of glutamine drives rampant bacterial overgrowth. Even typically beneficial microbes, such as Pseudomonas protegens CHA0 and Pseudomonas sp. Root68, triggered a sustained, low-level immune response, marked by heightened expression of defense-related genes.
However, the real danger comes when pathogens enter the mix. Without an intact barrier, mutant plants became highly susceptible to opportunistic infections. When challenged with Pseudomonas chlororaphis PK61 or Pseudomonas brassicacearum Root401 (opportunistic pathogens), they suffered stunted shoot growth and severe stress symptoms—essentially feeding their foes through uncontrolled nutrient leakage. Similarly, plants engineered to overexpress amino acid exporters, mimicking mild leakage, showed comparable vulnerability.
The findings underscore that the endodermis is more than a passive filter—it’s a dynamic gatekeeper essential for microbial balance and plant health. By tightly regulating internal nutrient flow, roots not only nourish themselves but also prevent their microscopic allies from turning into threats.
A New Paradigm for Root-Microbe Conversations
In conclusion, the study reveals a previously unrecognized pathway for root exudate formation—transient metabolite leakage—that complements known mechanisms such as membrane secretion and cell shedding. This finely tuned, spatially restricted nutrient control reframes our understanding of root-microbe dynamics, showing that today’s microbial communities may have arisen from yesterday’s fleeting nutrient pulses.
By pinpointing glutamine leakage as a key driver of microbial hotspots, the research highlights the importance of microscale resolution in studying plant-soil interactions and opens new possibilities for microbiome-informed breeding to enhance crop resilience. Ultimately, it portrays the root not as a passive interface, but as a vigilant manager—strategically nourishing its microbial allies while defending against opportunists—to safeguard plant health from beneath the soil.
Reference
Tsai, H. H., Tang, Y., Jiang, L., Xu, X., Dénervaud Tendon, V., Pang, J., . . . Zhou, F. (2025). Localized glutamine leakage drives the spatial structure of root microbial colonization. Science, 390(6768), eadu4235. doi: 10.1126/science.adu4235

