By YAN Fusheng
A type of novel biodegradable fibers, made from magnetic particles and the patient’s own blood, promises an immune-evading brain cancer therapy with minimal invasion.
Biodegradable fibers, made from magnetic particles and the patient’s own blood, offer a new way to fight brain tumors. (Graphic: AI generated)
A research team led by Dr. XU Tiantian from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, together with her collaborators from Shenzhen University and The Chinese University of Hong Kong, has developed magnetically controlled “blood robots” capable of navigating the brain’s fluid-filled crevices to deliver chemotherapy directly to tumors—all while evading the body’s immune defenses. Published in Nature Biomedical Engineering on May 1, the study introduces biohybrid blood hydrogel fibers (BBHFs) to take on one of oncology’s most vexing challenges: treating deep-seated brain tumors without invasive surgery or systemic drug side effects.
The Innovation: Blood Meets Magnetism
The BBHFs are crafted by mixing a patient’s blood with magnetic nanoparticles and clotting agents to form flexible, worm-like fibers. These fibers—just 1 mm thick and up to 5 cm long—can be injected near a tumor and steered through cerebrospinal fluid using external magnetic fields. Once positioned, a high-frequency magnetic pulse shatters the fibers, releasing encapsulated drugs like doxorubicin, precisely targeting wherever needed.
The system operates akin to a GPS-guided torpedo, where the patient’s own blood serves as both the delivery vehicle and drug carrier. This design minimizes rejection risks while leveraging the natural properties of blood to blend into the body’s environment.
Brain tumors, such as gliomas, are notoriously difficult to treat. Surgery often risks damaging critical neural pathways, while chemotherapy struggles to cross the blood-brain barrier. Current microrobots face immune system attacks or get swept away by fast-moving blood. Cerebrospinal fluid, however, flows 100 times slower than blood—a calmer environment for BBHFs to navigate.
In pig trials, BBHFs reduced tumor volumes by 60% compared to controls, with no immune response detected when using the animal’s own blood. By contrast, fibers made from donor blood triggered significant inflammation, underscoring the importance of personalization.
How It Works: From Lab to Brain
The process begins in the lab, where an experimental animal’s blood, or a patient’s blood in the future, is drawn and blended with iron oxide particles to create a magnetic slurry. This mixture is then injected into glass molds, hardening within minutes into flexible, worm-like gel fibers. Once prepared, doctors take the reins: using real-time X-ray imaging, they guide the fibers through the brain’s labyrinthine folds and fluid-filled cavities by manipulating rotating magnetic fields—like puppeteers steering marionettes with invisible strings. When the fibers reach their target, a magnetic “kill switch” activates, delivering a magnetic pulse as strong as 50-milliTesla at a frequency of 24 Hz, and shattering the structures into biodegradable fragments. The rupture unleashes a concentrated flood of chemotherapy drugs directly into the tumor, bypassing healthy tissue while avoiding the body’s usual defenses.
The fibers’ soft, flexible structure—similar to brain tissue—prevents damage as they squeeze through tight spaces. Their movement mimics earthworms, adapting to contours smaller than their diameter, which is impossible for rigid tools.
Crucially, this entire process can be visualized in real-time using X-ray fluoroscopy, allowing clinicians to track the BBHFs’ journey to the tumor. This is like having a GPS and live video feed for your internal medical robots.
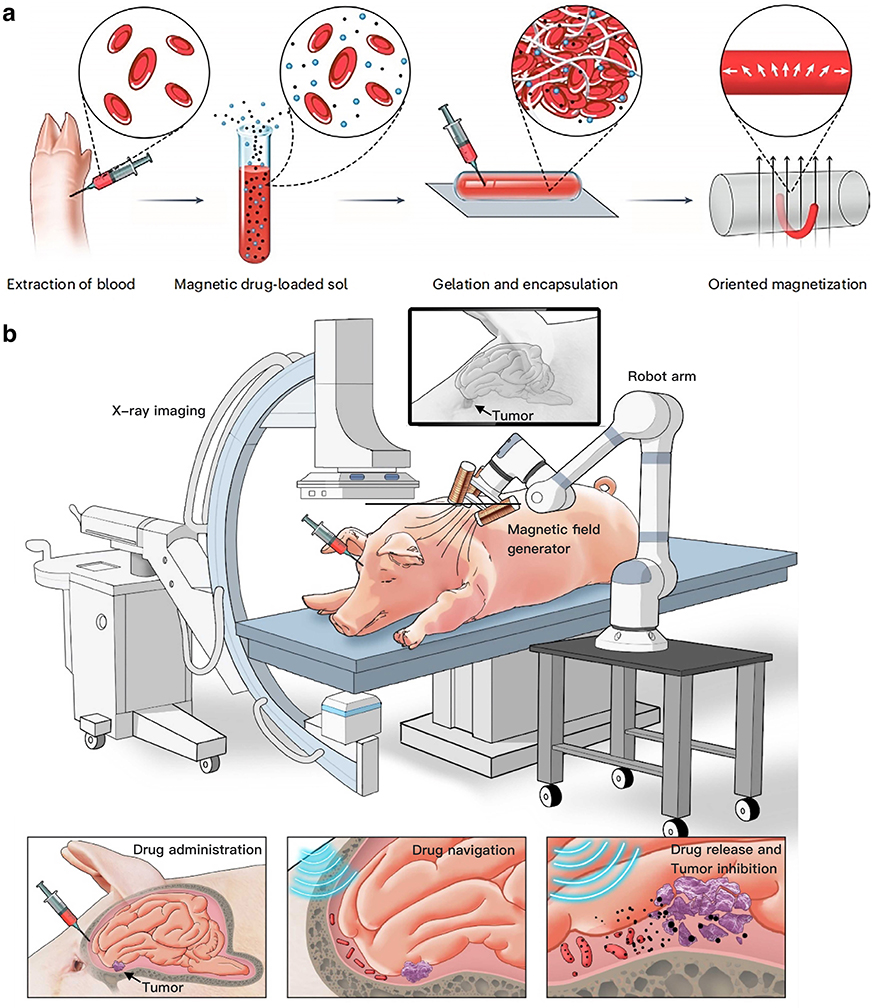
Schematic illustration of BBHF-mediated brain tumor therapy. (a) Fabrication strategy of biohybrid blood hydrogel fibers (BBHFs). (b) Therapeutic workflow: Modular assembly of BBHF-mediated brain tumor therapy, intracranial delivery via injection, active guidance to the tumor site, and localized drug release resulting in tumor growth inhibition. (Graphic: WANG Ben)
Future Horizons
The iron oxide particles used showed no organ accumulation in pigs, alleviating toxicity concerns. However, long-term effects of frequent magnetic exposure remain under study. The magnetic fields employed are comparable to those in MRI machines, ensuring alignment with established safety protocols.
While current tests focus on brain tumors, the team envisions BBHFs tackling spinal cancers or even clearing clots in stroke patients. Scaling production and refining magnetic controls for human trials are in their plans.
Reference
Wang, B., Shen, J., Huang, C., et al. (2025) Magnetically driven biohybrid blood hydrogel fibres for personalized intracranial tumour therapy under fluoroscopic tracking. Nature Biomedical Engineering. doi:10.1038/s41551-025-01382-z

